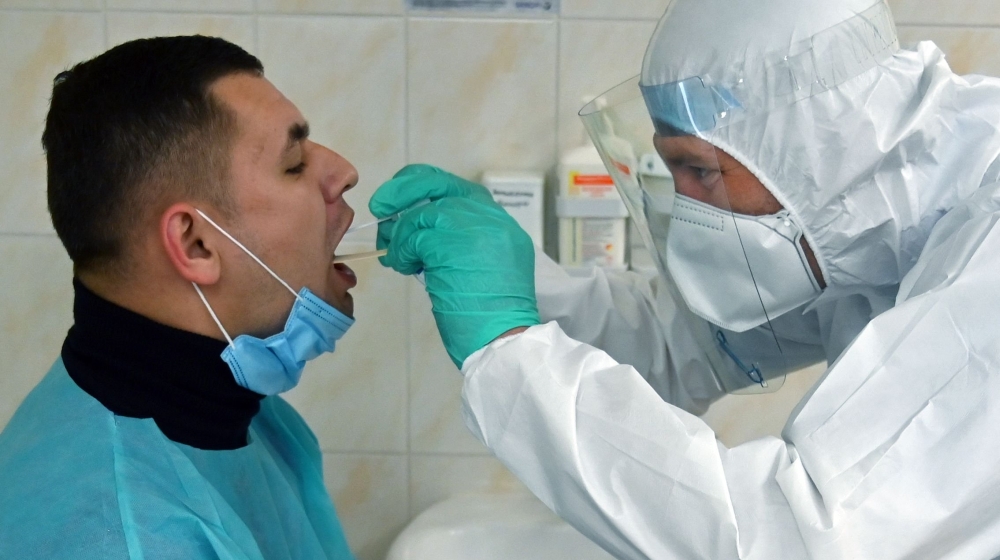
A Japanese drug company has begun the final stages of human trials for its anti-flu drug for treating coronavirus, it announced Tuesday.
Fujifilm’s Toyama Chemical branch makes a drug called Avigan, which is approved in Japan for stopping the flu virus from replicating in the body.
Researchers are of the opinion it may well have the same antiviral effect against the virus that causes COVID-19.
As per reports, Avigan, also known by its generic name, favipiravir, has fared well in its first two rounds of clinical testing, and the company has ramped up its production of the drug in anticipation that it could be the first proven treatment for the infection has now become a pandemic.
So far, it’s been approved by the Japanese equivalent of the Food and Drug Administration (FDA) to treat the flu, a common but deadly viral respiratory infection that kills some 500,000 people worldwide each year.
The company hopes the drug could work similarly against the current pandemic of coronavirus to how it interrupts the more common respiratory virus, flu.
Like the malaria drug being tested in the US, hydroxychloroquine, Avigan has the benefit of already being approved for other uses, meaning it will be available much more quickly than would be a wholly new, targeted treatment for coronavirus could be.
Fujifilm is already looking ahead to that possibility, and ordered an increase in production for Avigan in early March.
And it says that, pending approval to do so, Avigan will be distributed not just in Japan but around the world.